Our new publication in Lab Chip "Reliable and reusable whole polypropylene plastic microfluidic
- 2019年7月22日
- 讀畢需時 3 分鐘
已更新:2021年10月3日

With Antimicrobial susceptibility test (AST) as an important and representative example of application, this work demonstrates a practical method to fabricate microfluidic chips entirely using polypropylene (PP), and the benefits for potential broad real-world implementations.
Antimicrobial resistance (AMR) has become a major threat to modern medicine and it is primarily caused by the misuse or abuse of antibiotics. Antimicrobial susceptibility test (AST) is a promising way to help the optimal use of antibiotics and potentially reduces AMR. However, current phenotypic ASTs suffer from long turnaround time, while genotypic ASTs suffer from low reliability, and both are unaffordable for routine use. As a result, there is a great demand for developing rapid and accurate AST techniques.
New phenotypic AST methods based on microfluidics have been demonstrated to take only a few hours. However, they haven’t been translated into implementations mainly because of the well-known issues of surface fouling and leaching with the PDMS chip material, which is detrimental to a stable and reliable AST result, and the cost for making such cartridges that can be used only one time further limits the potential for commercial use. There is a vital demand of a new material to address these challenges and bring the conceptual advances to real implementations.
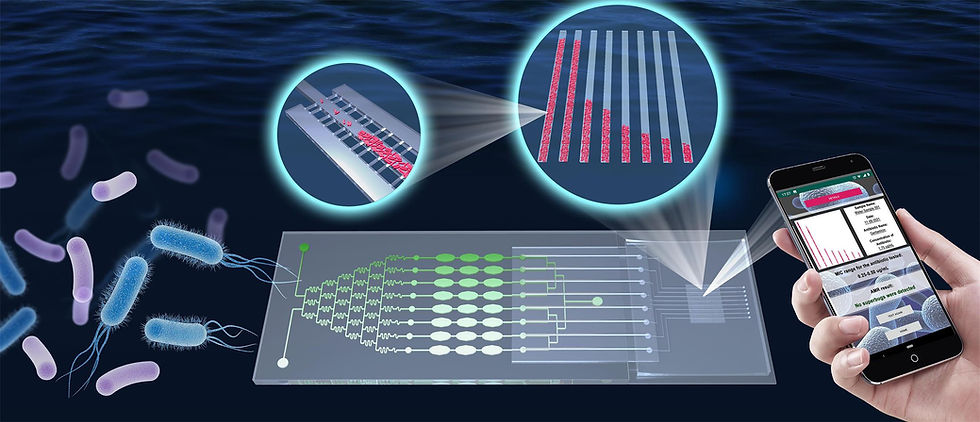
In this manuscript, we present a promising new material for microchips towards this need. We demonstrated a convenient yet powerful method to fabricate monolithic polypropylene (PP) microfluidic chips. The fabrication allows submicron resolution and quasi-3D structures, with the capability of mass-production at affordable cost. PP is the most commonly used material for anti-fouling labware such as vials, containers, caps, coatings, and pipette tips. Compared to PDMS, PP is cost-efficient, solvent-compatible, reusable, free from the absorption of hydrophobic drug molecules and water evaporation. But the reports of using PP to make microfluidic chips were very limited. A plausible reason was that there lacked a method to fabricate PP microchips with sufficient resolution at affordable cost; the commonly used hot-embossing method of standard soft lithography using PDMS masters to fabricate PMMA and PS chips (which are, however, prone to fouling) was unsuitable for microfabricating PP due to gas leaching problem because of the relatively higher melting temperature of PP.
Based on our modified soft lithography method, we demonstrate a reliable whole-PP microfluidic chip for rapid AST that shows several advantages compared to PDMS chips. The PP chips are more reliable and affordable than PDMS chips. Minimum inhibitory concentration (MIC) values of these antibiotics obtained were in accordance with the data from Clinical testing laboratory and Laboratory Standards Institute (CLSI), and showed better precision and reliability than those from PDMS chips. The cost of a PP chip is about 1/100 of a PDMS chip, not to mention the reusability which PDMS chips don’t have. Our system allows reliable tracking of individual cells and obtaining AST results within 1-3 hours, which is among the fastest phenotypic methods. Besides, with the aid of hydrodynamic simulation and experimental validation, our work employed specially designed 3-D shaped chambers in the microchannels for fast trapping of bacterium cells. This addressed the challenge of shear stress to bacterium culture in a novel design, which, compared to reported other designs, reduce hydrodynamic resistance and facilitate quicker operation, and conveniently realized high-throughput operation. In this regard, our design is also advantageous in terms of the speed and throughput of the analysis compared to existing methods.
Our PP chips provide a practical solution to upgrade the culture-based AST and benefit the battle against AMR through helping doctor prescribe effective narrow-spectrum antibiotics based on testing results. The new fabrication method for making whole-PP chips will also be useful for other applications where a reliable, solvent resistant, anti-fouling, yet affordable microfluidic chip is needed.
In conclusion, we believe our work has an immediate merit and a broad impact. The immediate merit is a new antimicrobial susceptibility testing (AST) that addresses a common problem of previous microfluidic AST methods and thus shows the best performance among reported phenotypic methods; the broad impact is the first report of a method to fabricate microfluidic chips using polypropylene (PP) with both sufficient resolution and affordable cost; the broad adoption of PP may address the common problems with PDMS and advance the broad application of microfluidic technologies through commercial implementations.
Update: this technology has been used in a subsequent work "“Barcode” Cell Sensor Microfluidic System: Rapid and Sample-to-Answer Antimicrobial Susceptibility Testing Applicable in Resource-Limited Conditions” which has been published in Biosensors and Bioelectronics. For details, please refer to:






















留言