Our research article “ “Barcode” Cell Sensor Microfluidic System" in Biosensors and Bioelectronics
- 2021年10月3日
- 讀畢需時 3 分鐘
已更新:2021年10月4日
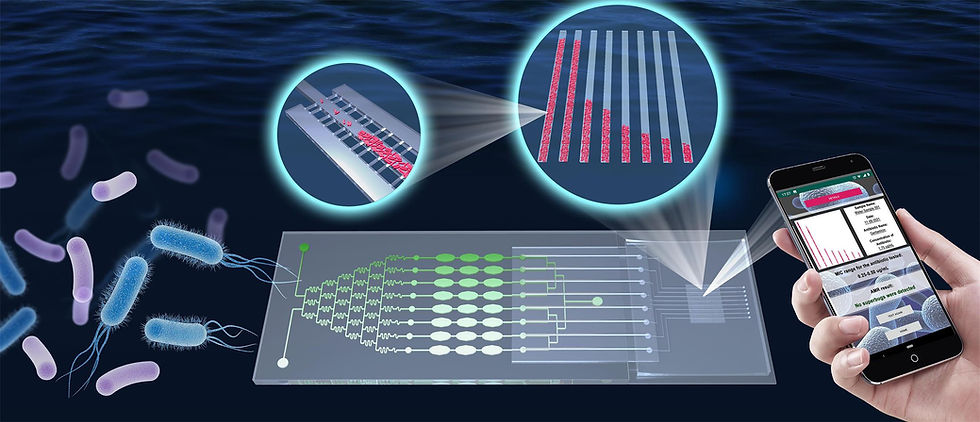
Antimicrobial resistance (AMR) is growing into a major threat to modern medicine, and efforts should be made in multiple aspects to address this grand challenge. Antimicrobial susceptibility testing (AST) is an important tool promising to provide accurate bacterial information, such as minimal inhibition concentration (MIC), which determines the optimal use of antibiotics and potentially reduces AMR. One major remedy is to reduce the misuse or abuse of antibiotics, especially wide-spectrum ones prescribed without a solid clue of whether and which microorganism is causing an infection. Many teams including my own team, have made continued efforts in this field (e.g., Lab Chip 2016, ChemPlusChem 2017, Trends Biotech 2017, Lab Chip2019, etc.). These novel antimicrobial susceptibility testing (AST) methods are being developed to help physicians provide more personalized therapies.
On the other hand, however, there has yet been sufficient tools to deal with AMR issues outside well-equipped healthcare facilities. These issues actually cover a wide range of situations, e.g., frequent surveys for the safety of water, food, and public facilities; an urgent survey of massive samples during a pandemic; or any AMR test in low-income countries. The majority of novel clinical AST methods realized thus far have a variety of drawbacks when used for such surveys, e.g., high cost, dependence on microscopic analysis, and required use of expensive instrumentation. A more reasonable strategy would be to screen samples via routine onsite testing, and then send any samples suspected to contain AMR bacteria for advanced testing. Thus, a resource-independent and cost-efficient AST method that can rapidly do so for a large number of samples is of great demand. In particular, the lack of a microscope-free method that can analyze very small amounts of bacteria has been a major hurdle to enable rapid culture-based AST in resource-limited conditions.
To meet this requirement, we invented a novel “barcode” cell sensor based on an adaptive linear filter array as a fully automatic and microscope-free method for counting very small volumes of cells, wherein suspended cells concentrate into microbars through the filtration effect of nanochannels at the channel sides (patent pending). We combined this sensor with an on-chip culture that takes much less time than standard methods, thereby realizing a low-cost and high-throughput platform for portable AST, from which results can be obtained through a cell phone. Our “barcode” cell sensor has shown enormous potential for testing samples in resource-limited conditions, without high demand of operation skills due to its microscopy-free analysis, affordability, portability, high throughput, and user-friendliness, making it a powerful weapon in the fight against AMR.
We provided a detailed discussion on the mechanism of our innovative adaptive linear filter and the “barcode” cell sensor, which could be of interest to readers in sensors, hydrodynamics, microbiology, microfabrication, microfluidics, etc. On the other hand, the outstanding functionalities of our system in microscope-free, rapid, and quantitative AST could be of interest to readers in antimicrobial resistance, environmental analysis, clinical microbiology, etc. This article is published in Biosensors and Bioelectronics which can be accessed through the following link.
P.S., this system is designed for the low-cost/onsite preliminary screening stage of our two-tier design for advanced microfluidic AST. The second tier is for high-performance testing in laboratory, which is discussed in our another post, which can be found here: Hong Kong university have invented a faster, cheaper and more accurate way to identify how resistant bacteria are to antibiotics
News reports to this work:
HKBU
HKBU invents novel cell sensor for rapid and low-cost screening of drug-resistant bacteria (press release)
Xinhua Finance Agency
Bizwire Express
The Reporting Today
etnet
A blog feature article in Chinese:






















留言